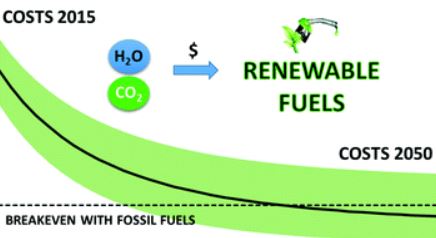
The future of solar fuels: when could they become competitive?
Solar energy driven processes with H2O and CO2 as basic feedstocks can produce “solar fuels” that could substitute their fossil based counterparts. This article summarizes the main findings of a techno-economic analysis of systems that can generate different types of fuels with renewable energy as starting point. These “renewable fuels”
New Study: Global Energy System based on 100% Renewable Energy
The new study by the Energy Watch Group and LUT University is the first of its kind to outline a 1.5°C scenario with a cost-effective, cross-sectoral, technology-rich global 100% renewable energy system that does not build on negative CO2 emission technologies. The scientific modelling study simulates a total global energy
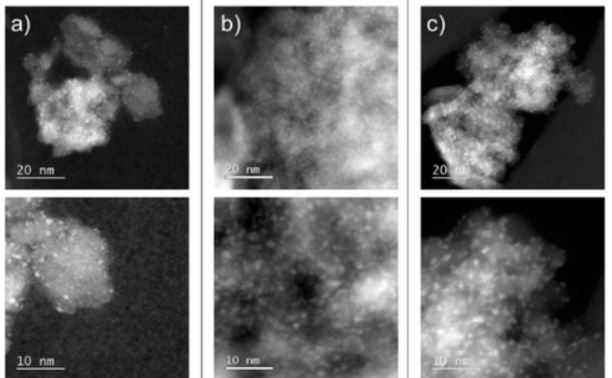
Collective photothermal effect of Al2O3-supported spheroidal plasmonic Ru nanoparticle catalysts in the sunlight-powered Sabatier reaction
Plasmon catalysis is an interesting technology concept for powering chemical processes with light. Here, we report the use of various Al2O3-supported Ru spheroidal nanoparticles as catalyst for the low-temperature conversion of CO2 and H2 to CH4 (Sabatier reaction), using sunlight as energy source. At high loadings of Ru spheroidal nanoparticles
“SPOTLIGHT solar fuels”: a disruptive photonic technology to create carbon neutral fuels
A new H2020 project that will develop innovative photonic devices for highly efficient, sunlight-fueled chemical processes. The sun is a valuable source of energy. It can be used to produce electricity but it can also be stored into complex chemical molecules: the solar energy allows converting feedstocks such as carbon dioxide
A Study on the Contribution of Laser Illumination
The interaction between plasmonic metal catalysts and visible light can be exploited to increase their catalytic activity. This activity increase results from the generation of hot charge carriers or hot surfaces, or a combination of both. We have studied the light-induced Suzuki-Miyaura cross-coupling reaction of bromobenzene and m-tolylboronic acid using
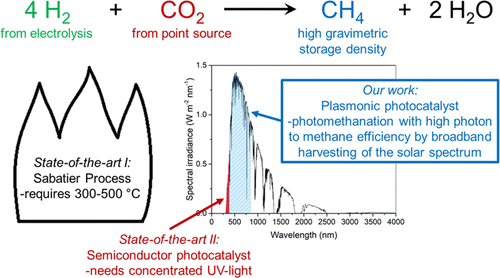
Sunlight-Fueled, Low-Temperature Ru-Catalyzed Conversion of CO2 and H2 to CH4 with a High Photon-to-Methane Efficiency
Methane, which has a high energy storage density and is safely stored and transported in our existing infrastructure, can be produced through conversion of the undesired energy carrier H2 with CO2. Methane production with standard transition-metal catalysts requires high-temperature activation (300–500 °C). Alternatively, semiconductor metal oxide photocatalysts can be used,