Sunlight-Fueled, Low-Temperature Ru-Catalyzed Conversion of CO2 and H2 to CH4 with a High Photon-to-Methane Efficiency
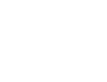
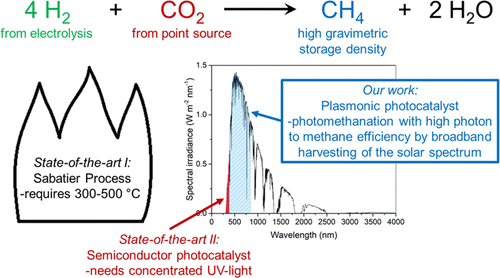
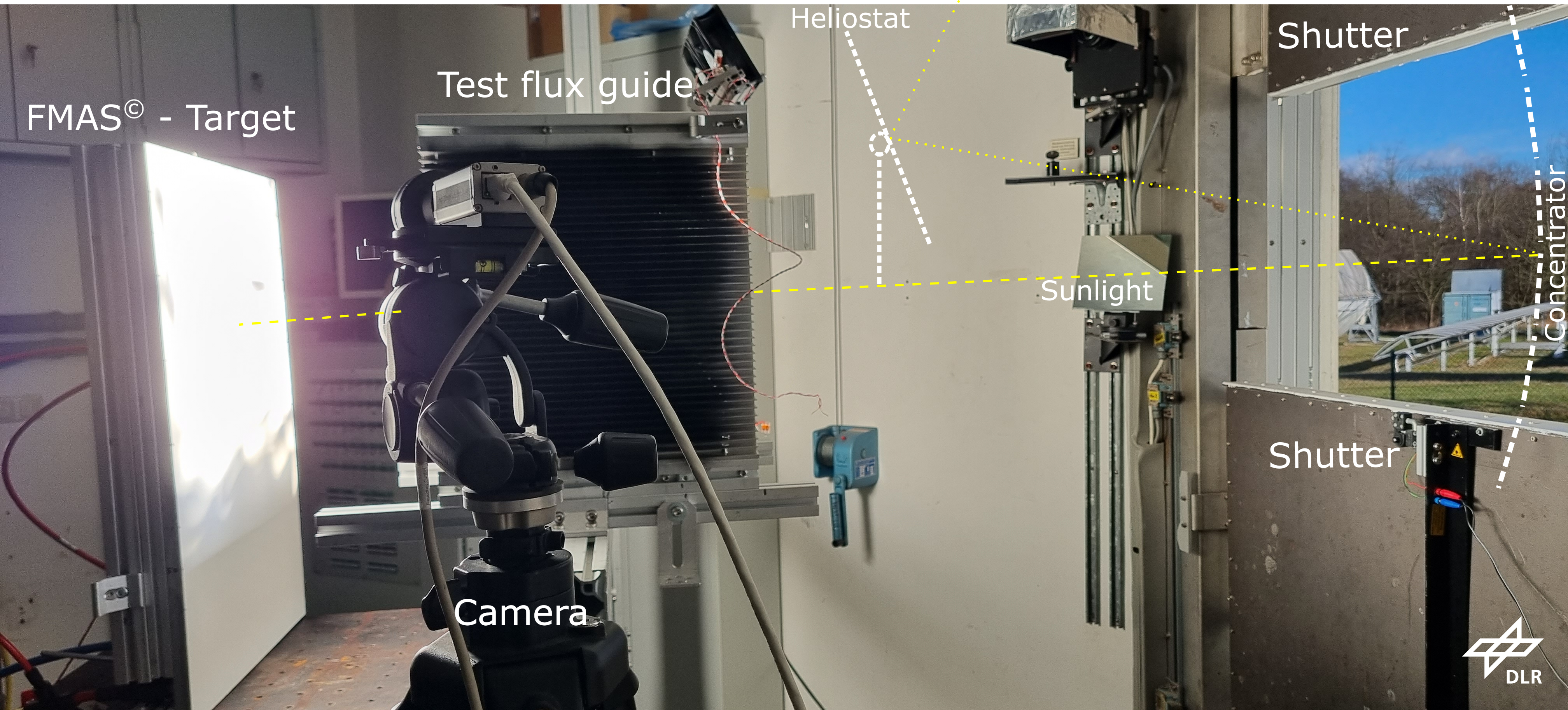
Methane, which has a high energy storage density and is safely stored and transported in our existing infrastructure, can be produced through conversion of the undesired energy carrier H2 with CO2. Methane production with standard transition-metal catalysts requires high-temperature activation (300–500 °C). Alternatively, semiconductor metal oxide photocatalysts can be used, but they require high-intensity UV light. Here, we report a Ru metal catalyst that facilitates methanation below 250 °C using sunlight as an energy source. Although at low solar intensity (1 sun) the activity of the Ru catalyst is mainly attributed to thermal effects, we identified a large nonthermal contribution at slightly elevated intensities (5.7 and 8.5 sun) resulting in a high photon-to-methane efficiency of up to 55% over the whole solar spectrum. We attribute the excellent sunlight-harvesting ability of the catalyst and the high photon-to-methane efficiency to its UV–vis–NIR plasmonic absorption. Our highly efficient conversion of H2 to methane is a promising technology to simultaneously accelerate the energy transition and reduce CO2 emissions.